In order to survive, a cell must be able to understand its environment. This is true whether the cell is a single-celled organism or part of a larger, more complex multicellular organism. Cells communicate with their environment through a process called signaling. Cell signaling is how the cell collects information and then responds with an action at the correct time. Signaling is the initial event associated with many key cellular functions, from the correct timing of cell division, to the decision to migrate in a particular direction, and even to whether a cell needs to go through programmed cell death. So many of the cellular events we explore in biology are dependent on signaling to happen correctly. Not only that, but many of the concepts covered in upper-level biology courses are, at their hearts, studies of how the cell receives information and responds to it. For example, developmental biology, sensory perception, endocrinology, and even physiology will make much more sense if you have a foundational understanding of how signaling works.
Thus, our focus in this chapter is to dissect the process of signaling and take a look at the parts of a signaling cascade and some of the commonly recurring themes and patterns. That way, when you encounter any pathway in any context, you will have the ability to “read it” through and identify the patterns within it to correctly interpret the outcomes of that pathway.
While signaling is a beautiful coordination of cellular events, it also is a very complex process. It can be very confusing to keep track of what the cell is doing at any given time due to the sheer number of signals, the overlap in signaling pathways, and sometimes even the competing signaling events occurring simultaneously. Additionally, a wide range of cellular “behaviors” are mediated through a small set of extracellular signals. Thus, the way that a cell responds to a specific extracellular signal will depend on what genes are being expressed in the cell at that specific moment in time. For example, acetylcholine is an extracellular signal that has different effects in different cell types. It is released at a neuromuscular junction by neurons to promote muscle contraction. In cardiac pacemaker cells, it signals that the heart rate should lower, so the pacemaker cells fire at a decreased rate. Finally, salivary glands also respond to acetylcholine by increasing the synthesis and secretion of saliva by the endomembrane system. Each of these scenarios is the result of different modes of release of the acetylcholine, different receptors receiving it on the cell surface, and also differential expression of the internal components of the acetylcholine pathway.
Our focus for this topic is to lay some groundwork for understanding signaling by looking at the general principles that underlie cell signaling. Then in the next topic, we’ll look at some examples of actual mechanisms that are commonly used in cell signaling. These tools should allow you to interpret any signaling pathway in the future and begin to understand how they mediate a response.
The video below (Video 07-01) gives a quick overview of the mechanism of cell signaling and the terminology you will need to understand this process.

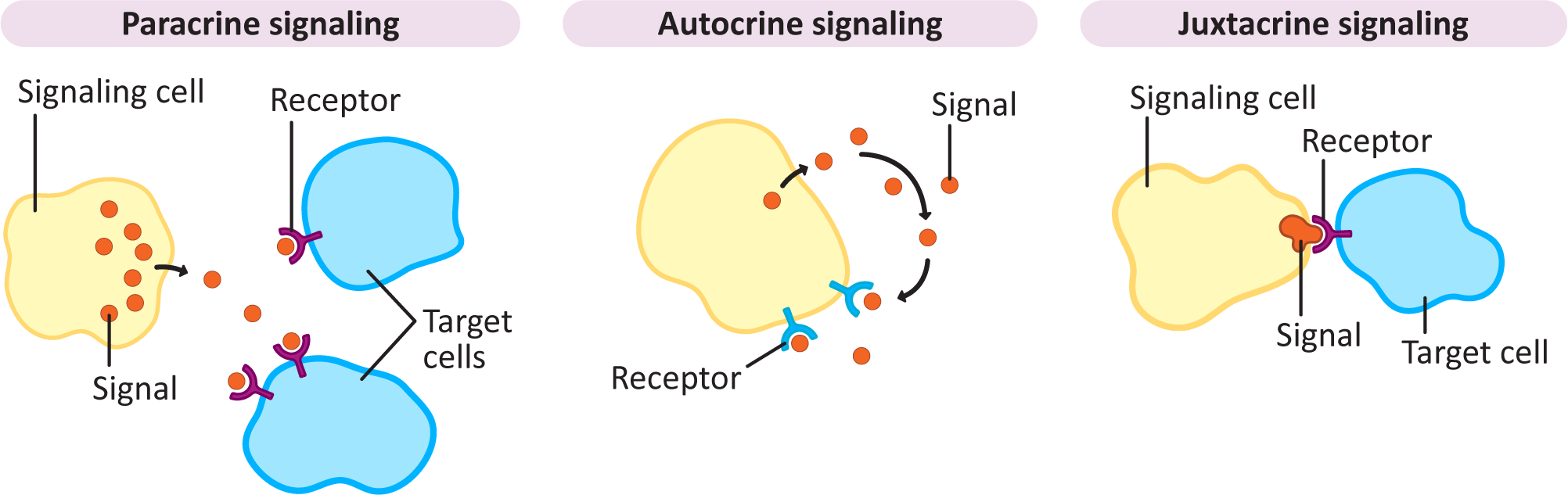
In many ways, signaling at closer range is much easier than long-distance signaling, as the signal can be released into the extracellular space and simply left to diffuse. This is one of the most common types of signaling in development. Plants, algae, and fungi don’t really have any long-distance options that are as efficient as the bloodstream or neurons, so for them, almost all signaling is local and diffusion based. In addition, there are a number of examples of cells that release their own signaling molecules and then detect them with receptors. Here we look at the three types of short-range signaling in more detail. They can all be observed in Figure 07-02, below.
Paracrine signaling is considered to be a local signaling mechanism. The ligand is released into the extracellular space, and it diffuses through the extracellular matrix to be picked up by nearby receptors.
Autocrine signaling is when the signal is both released and received by the same cell. It is considered a type of paracrine signaling, since the signal must diffuse to the receptor through the extracellular environment.
Finally, juxtacrine signaling isn’t very common overall compared to the other kinds of signaling. It’s also known as contact-dependent signaling, which gives you an idea of what is involved in this form of signaling. In this case, the cell that receives the signal comes into direct contact with the one that is sending out the signal. Most commonly, this would mean direct cell-to-cell contact, but it may also be an interaction between a cell-surface receptor and a glycoprotein of the extracellular matrix.
The most famous example of cell-to-cell juxtacrine signaling is the Notch-Delta signaling cascade, which is essential to embryonic development in animals. The receptor (Notch) is a plasma membrane protein on one cell, and the ligand (Delta) is on the other. When they bind, the cytosolic side of the Notch receptor is cut off and becomes a transcription factor that then enters the nucleus. As a result, the transcription factor activates gene expression, and the “fate” of this cell is permanently and irreversibly changed. Notch-Delta signaling is extremely important in development, when cells are gaining their identities.
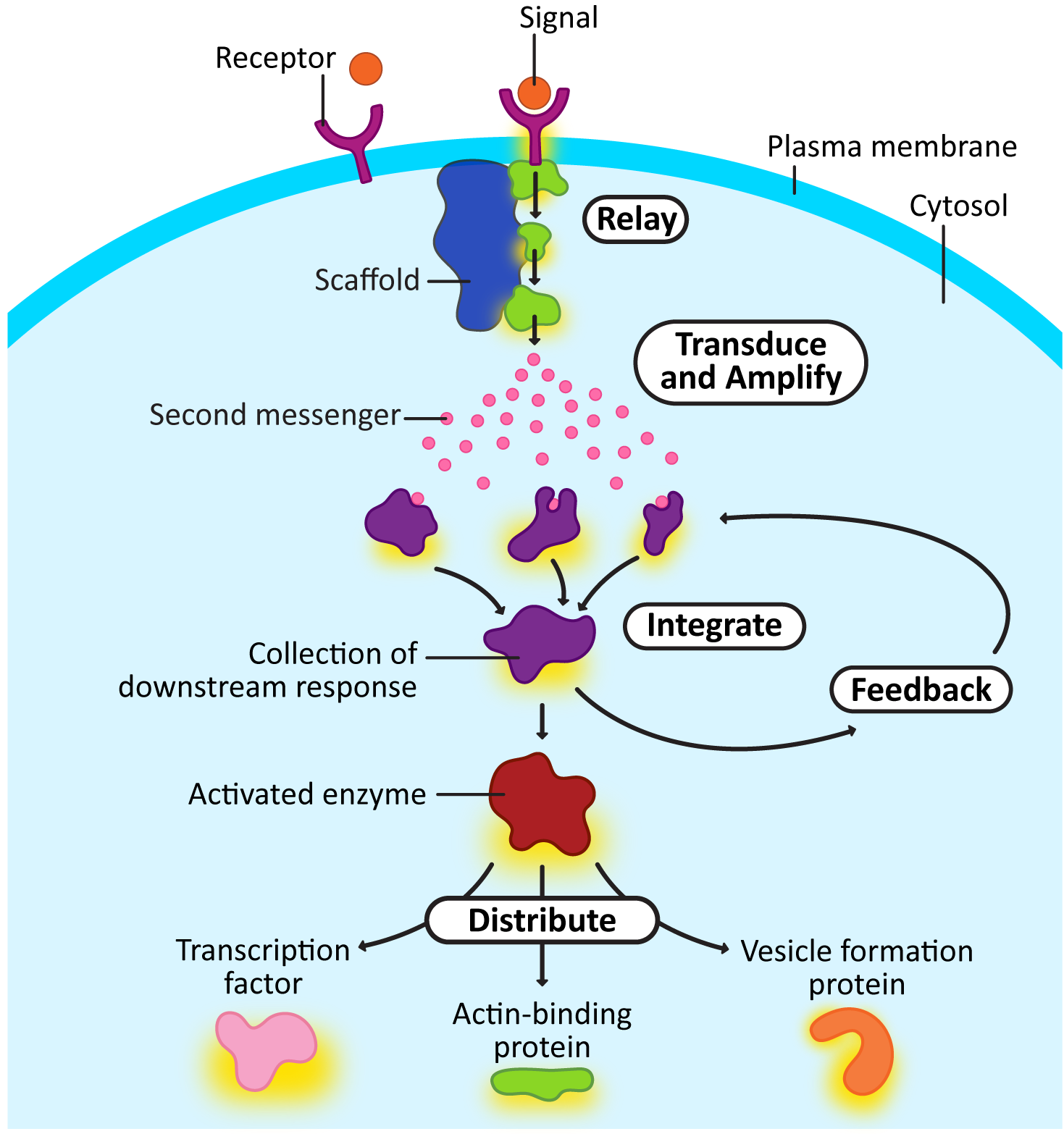
Here’s a quick explanation of each of the intracellular signaling steps from Figure 07-03. Later, we’ll look at some of the more famous examples of proteins that perform these roles. Remember that not all of these are present in every signaling cascade, and sometimes an individual element could be performing more than one function.
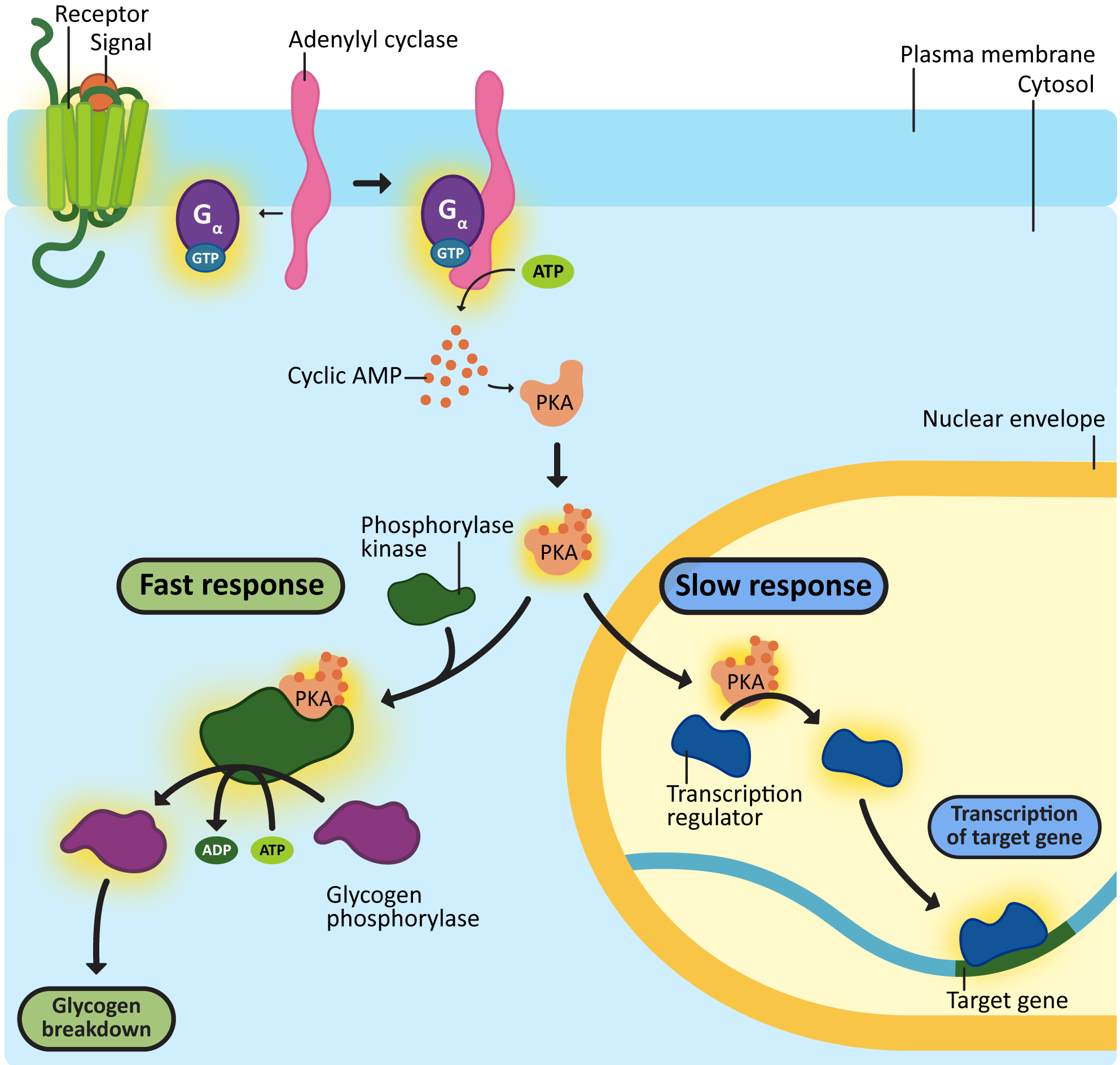
The cellular responses at the end of the signaling cascade usually fall into two categories: fast responses and slow responses. The difference between these is very simple. Cellular responses that require protein synthesis will always be slower than responses that rely on preexisting proteins, which can be simply altered.
Often, a signaling event will result in an overall change in cellular identity. Changing the identity of a cell will require a number of changes in gene expression: some proteins will need to be synthesized, while others will likely need to be removed from the cell. For example, most of the signaling events involved in development will cause changes in gene expression, as cell differentiation is a key step in embryonic development. As a secondary example, the immune system also uses alterations in gene expression so that antibodies can be produced in response to a pathogen or vaccine. In order to change gene expression, signaling cascades cause the activation or deactivation of transcription factors or proteins that affect chromatin structure (i.e., histone or chromatin-modifying enzymes). As we discussed in Chapter 3, proteins that influence histones and/or chromatin will impact the accessibility of the DNA, thus impacting the expression of genes in that area. The end result will be changes in the protein composition in the affected cell.
Alternatively, extracellular signals can stimulate signaling cascades that result in shorter-term changes in cellular function by altering preexisting proteins. A very good example of this, which we will explore in detail in Chapter 8, is mitosis. When mitosis is activated, the cell is vulnerable, so this process must be completed quickly. Most of the proteins involved in rearranging the cytosol for mitosis already exist before mitosis starts. They are present in the cytosol, waiting for the signal to initiate cell division. Once the signal is perceived, these preexisting proteins are modified as a result of the signaling cascade. This causes a rapid shift in cellular physiology, and the cells progress through mitosis as efficiently as possible.
So far in this chapter, we have discussed how signaling cascades are activated. In some cases, this is the end of the story, as the signal never needs to be turned off. An example of this would be the Notch-Delta signaling pathway, which changes the cell permanently. It doesn’t really need to be “turned off” in the traditional sense. Another great example would be apoptosis. The signaling cascades of apoptosis lead directly to the death of the cell, so once apoptosis is activated, there is no cellular mechanism to deactivate it.
On the other hand, a great many signaling events need to stop eventually so that the cell can go back to “normal.” Mitosis is a good example, as the signaling that started mitosis must be stopped in order for mitosis to end. Another good example is sensory perception. Every single one of our senses is controlled through signaling. If we can’t stop receiving and responding to the signal, then we can’t go back to normal so that the signal can be received again. Imagine if our pain receptors never turned off once they were turned on!
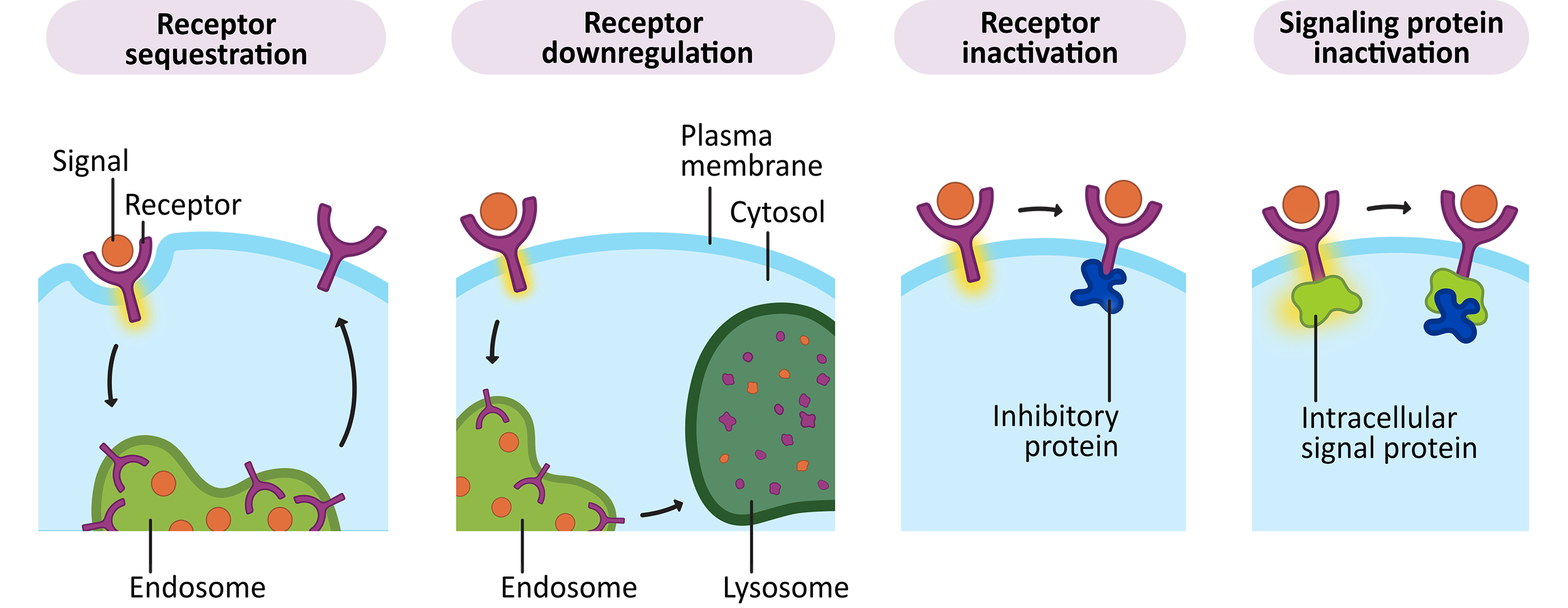
Figure 07-04 illustrates the most common ways that signaling cascades are stopped. Quite often, cells deactivate signals by inhibiting, or removing, the receptor. In some cases, it is an internal component that is deactivated.
Some of these changes are permanent. An example of permanent deactivation occurs in receptor downregulation, where the receptor is brought into the cell via endocytosis and then degraded in the lysosome. However, many other deactivation steps are reversible. Receptor sequestration pulls the receptor off the cell surface so that it can’t bind to a signal for a period of time but the receptor isn’t destroyed. In both receptor inactivation and signaling protein inactivation, one of the proteins that forms part of the signaling cascade is inactivated in some reversible way (most likely by phosphorylation and/or binding to an inhibitory protein).
Deactivation of signaling cascades is usually controlled via negative feedback loops. This means that the activation of the signaling cascade produces products that help shut down the signaling cascade as well.
Before we move on to the next topic, we should take a moment to discuss the way that scientists write out signaling cascades and how to interpret them.
As you likely have seen in the figures here or elsewhere in your course notes or the internet, signaling cascades are often written out with the proteins lined up and connecting symbols between them. Two important symbols are used in between the proteins to identify how they impact each other:
We often discuss the steps of a signaling cascade as being upstream or downstream of each other. So for example, the signal binding to the receptor is upstream of the cellular response. Conversely, the response will be downstream of ligand binding. These terms and symbols are important for interpreting the diagrams used to represent signaling pathways in textbooks and papers. Video 07-03 provides further explanation.
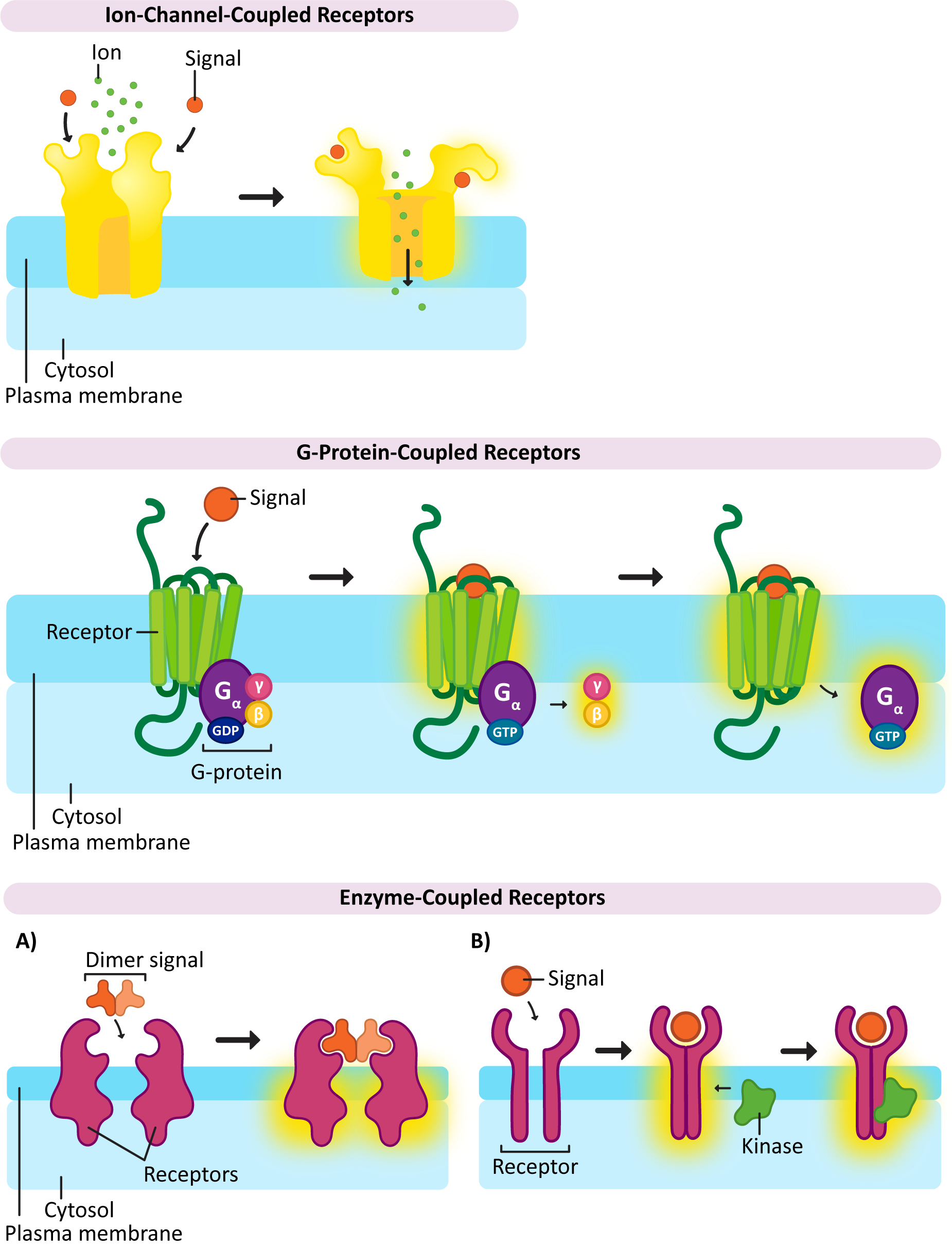
There is one important example of a ligand-gated ion channel that we will be discussing a bit later, and that’s the one used to release calcium into the cytosol from the ER. Remember that calcium is an important second messenger in cell signaling and is often used to amplify and spread the response throughout the cell. The ER-localized calcium channels that are used in this process open to a specific signal as well. In this case, another second messenger is used, IP3, which is released when the phospholipid, PIP2, is cleaved as part of a signaling response. We will see how this works a bit later in this topic.
The next group of receptors we’ll look at is G-protein-coupled receptors, or GPCRs for short. This type of receptor is by far the most common in animals. They are heavily involved in animal sensory perception, especially our sense of smell. Humans have over 700 GPCRs in total, most of which are dedicated to smell. On the other hand, a mouse, which has a much sharper sense of smell than we do, has over 1,000 different GPCRs involved in its sense of smell alone!! Even better sniffers, like the basset hound and grizzly bear we talked about earlier, are thought to have an even higher number of olfactory GPCRs.
GPCRs are a very large family of proteins that includes many subfamilies with specific functional differences. We won’t get into all of that detail here (it fits better with an upper-level signaling textbook), but we can identify some important characteristics of GPCRs that are common across the family. For example, the GPCR itself is almost always a single polypeptide chain with seven transmembrane domains that cross the membrane as alpha helices. This is considered to be a characteristic structural feature of these receptors. GPCRs have a large pocket for ligand binding, which allows for a lot of variation. Thus, the ligands that can bind to GPCRs are also quite variable. This is why they are such a good candidate to manage our sense of smell—they most commonly bind to small organic molecules rather than peptide hormones. Some of the light receptors on our retinas are GPCRs as well.
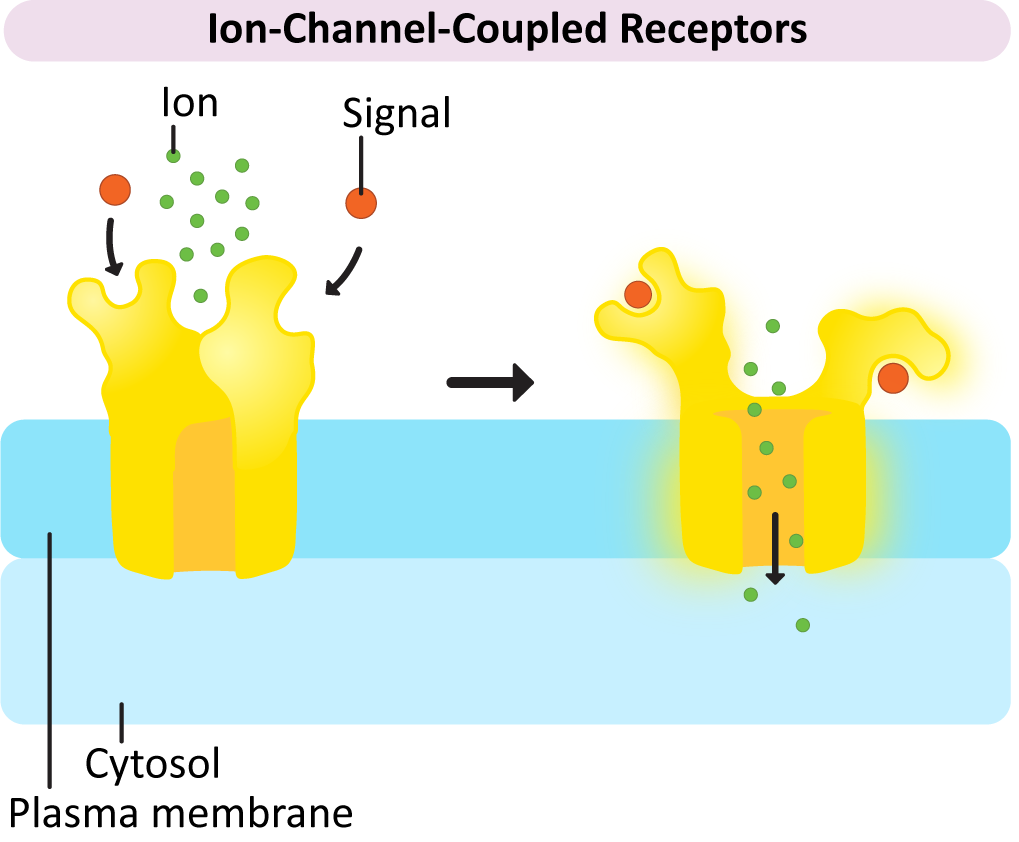
Once again, the name of this type of receptor tells you something about it. The receptor itself works in conjunction with another set of proteins called G-proteins. The “G” in G-protein stands for GTP, as GTP is required to activate the G-proteins and allows a response to be generated. G-proteins also come in a variety of flavors to match up with the GPCRs that they work with.
Despite variation in the G-protein family, they also have many similarities:
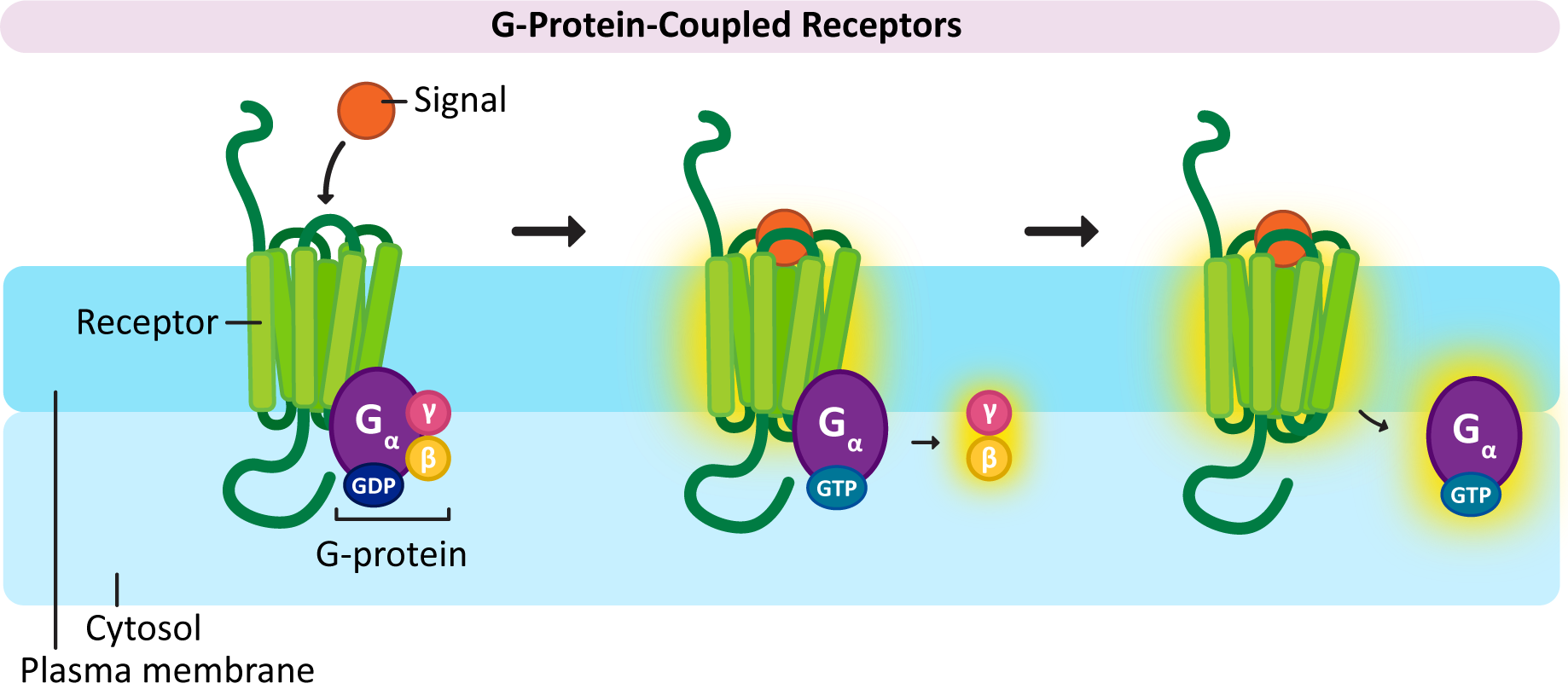
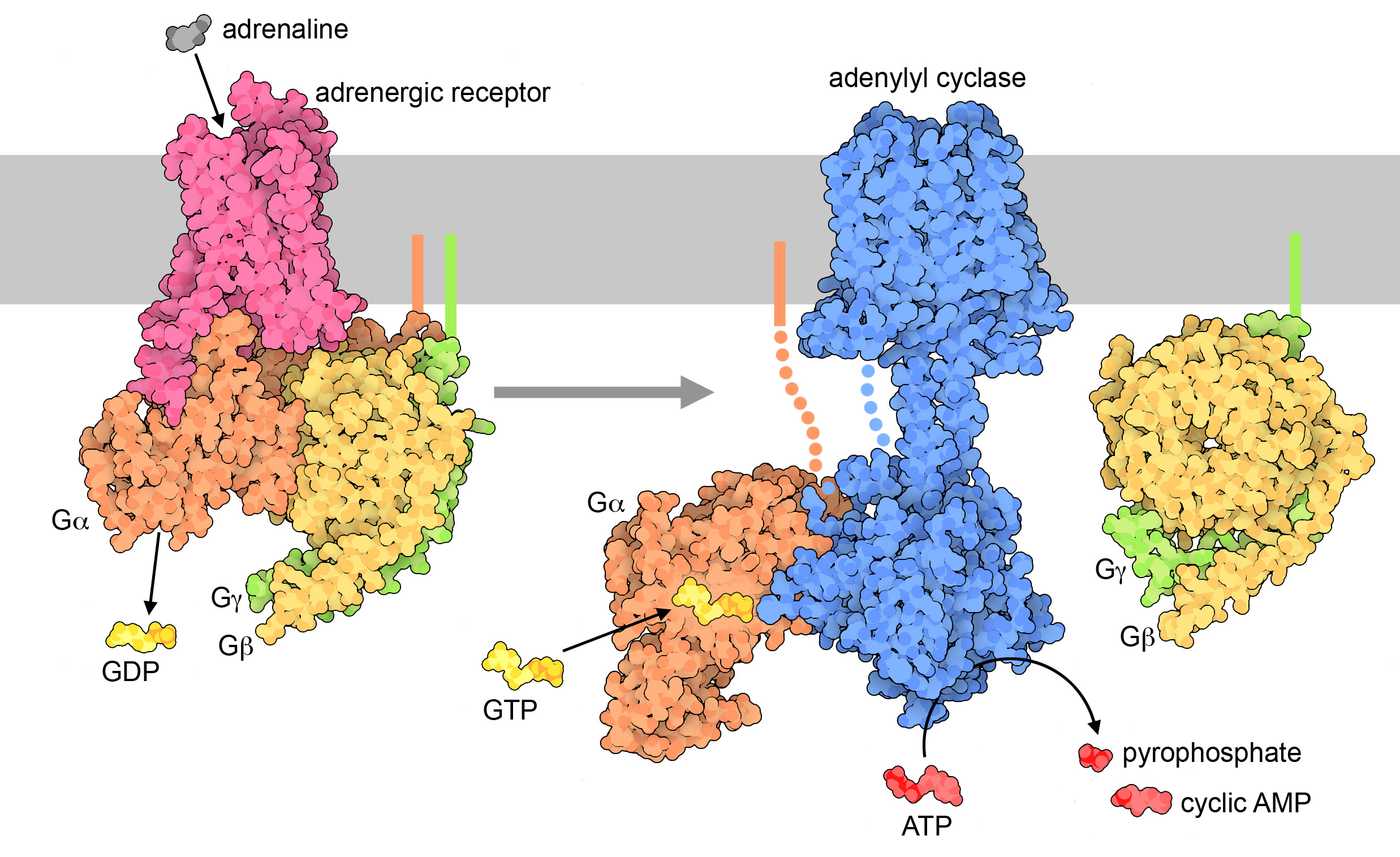
In a nutshell, here’s how GPCR activation works (Figure 07-07):
Now that we’ve discussed how the GPCR is activated and deactivated, we turn our discussion to an important downstream signaling molecule commonly used in GPCR signaling cascades: the production/release of second messenger molecules. We’ll specifically discuss the two most common second messengers that are activated by G-proteins: cyclic AMP and IP3/DAG.
Cyclic AMP, or cAMP, as it is often abbreviated, is a modified version of adenosine monophosphate (AMP). cAMP is made from ATP, a molecule you should know well that is extremely abundant in the cell. Figure 07-08 shows the structural changes required to interconvert ATP, cAMP, and AMP.
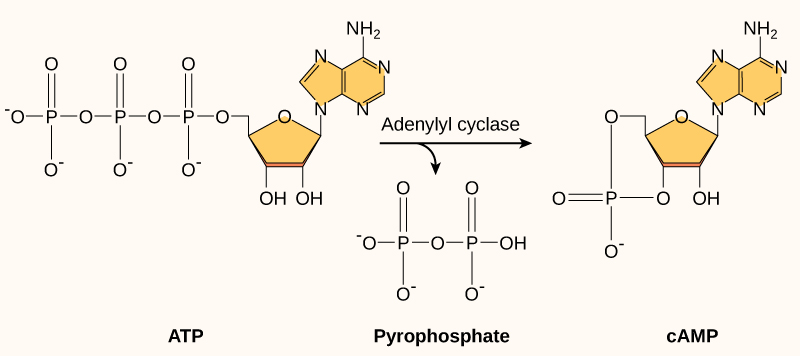
Cyclic AMP is produced when a plasma membrane–bound enzyme, adenylyl cyclase, is activated by the Gα subunit of the activated G-protein (Figure 07-09). Cyclic AMP is then capable of moving throughout the cell and interacting with any enzymes that have binding sites for it. cAMP is a short-lived molecule—its half-life in the cell is less than 1.5 minutes. An enzyme known as phosphodiesterase breaks it down so that the cellular response is kept short. Figure 07-09 and Video 07-05 (below) review the activation of GPCRs and discuss the production of cAMP.
DAG and IP3 are another set of common second messengers. They are always produced together, as they are two parts of the same initial phospholipid (called PIP2, which is a generic term for a phosphatidyl inositol phosphate with two phosphates added to the inositol sugar). PIP2 gets split in two by an enzyme known as phospholipase C (PLC) Figure 07-11) to produce the two second messengers. PLC is activated by a Gβγ protein complex. While the breadth of lipid function in cells doesn’t often get discussed at the introductory level, it is worth noting that there are a number of membrane lipids, especially phospholipids, that have a key role to play in cell signaling and membrane identity.
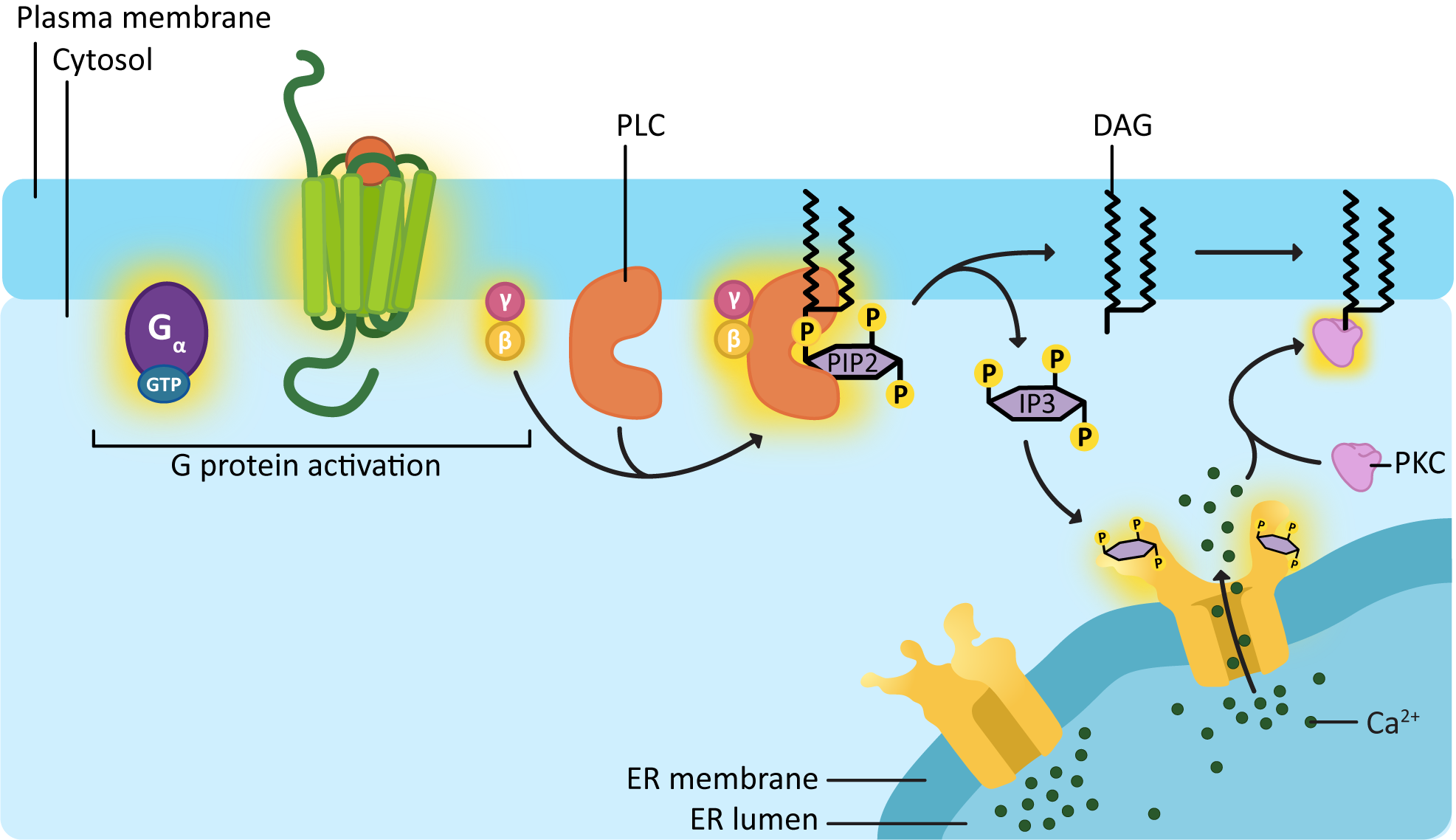
As illustrated in Figure 07-11, phospholipase C clips the soluble “head” portion of the phospholipid from the glycerol backbone and lipid tails. The head portion is a specific type of six-carbon sugar, with three phosphate groups on it, called inositol 1,4,5-triphosphate, or IP3 for short. The hydrophobic “tail” portion that is left in the membrane is now a molecule known as diacylglycerol, or DAG. Both of these molecules will be used to activate further components of the signaling cascade. DAG will diffuse laterally through the membrane and act as a docking site for a protein known as protein kinase C (PKC). IP3 is now soluble, so it can diffuse through the cytosol and bind to and activate its downstream targets.
One important protein that IP3 can bind to is a ligand-gated ion channel in the ER. This ligand-gated channel is responsible for releasing calcium ions, another important second messenger in the cell, into the cytosol. Much like cAMP, calcium can flood the cytosol very quickly, producing a rapid change in the membrane potential of the plasma membrane, among other things. Calcium waves that rapidly spread through the cell are a key event in many cellular processes, such as muscle contraction and oocyte fertilization.
The released calcium can also interact with many different targets in the cytosol. In Figure 07-11, we see that PKC requires calcium to bind in order for it to become active. So PKC activation requires both the docking site, produced by DAG, and the calcium released by IP3 before it can be activated. Activated PKC will then be able to phosphorylate a number of specific targets to generate change in the cell.
Eventually, the calcium is pumped back into the ER using different ion channels than those that were opened during calcium release. The new channels are opened by the presence of calcium in the cytosol, which makes this an excellent example of a negative feedback loop, where the signaling event activates the process that will also deactivate the signal.
Incidentally, calcium is by far the most commonly used second messenger in plants. Also of interest is that GPCRs are not nearly as common in plants as they are in animals. The most commonly used receptors in plants are receptors coupled to serine/threonine kinases. Ser/Thr kinases are enzymes, so these are a type of enzyme-coupled receptor. This leads us very nicely to the last type of receptor we need to discuss.
This final set of receptors that we need to discuss is much more structurally variable compared to GPCRs, but they all have one very important thing in common: when the ligand binds to the receptor, an enzyme known as a kinase is activated. Kinases add phosphate groups to other proteins. We’ve seen many examples of this kind of enzyme throughout this textbook (specific examples include PKA and PKC from the previous section). There are also enzymes that remove phosphate groups, which we call phosphatases.
Phosphate groups can only be added to proteins at specific sites—not all R groups on an amino acid are capable of having phosphate groups added. Kinases fall into two major categories based on the amino acid R groups they modify:
The enzyme-coupled receptors themselves will either
We won’t really differentiate between the types (integrated kinase or associated kinase) here, as there really isn’t a whole lot of difference between them from a functional standpoint. Additionally, for the most part, the examples we’ll see will be receptor tyrosine kinases (RTKs for short), where the kinase acts on tyrosine and is an intrinsic function of the activated receptor. Remember that there are also receptor-associated Ser/Thr kinases. Ligands for these kinds of receptors are often small peptides, such as growth hormones and insulin.
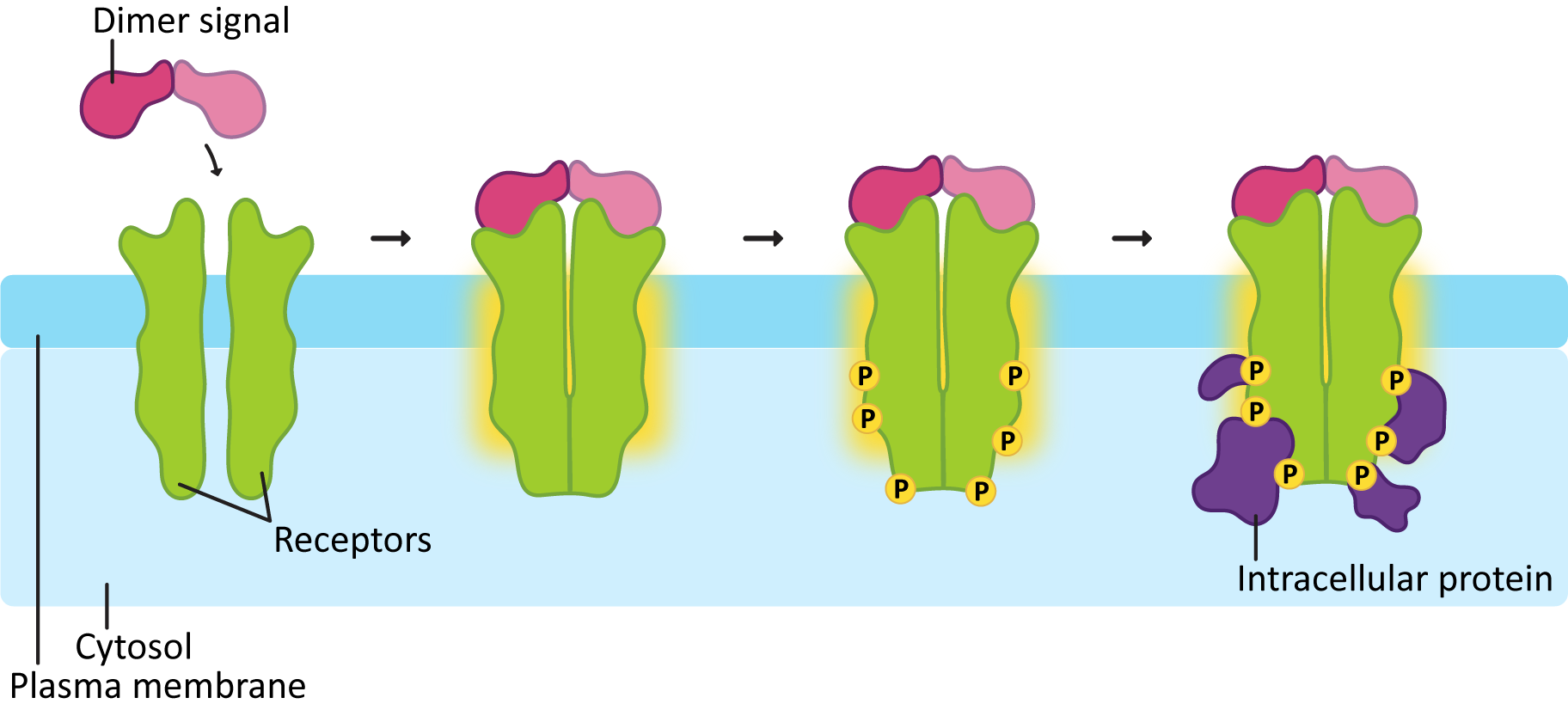
A few important points about RTKs are highlighted by Figure 07-12:
Much like GPCRs, there are both membrane-bound and soluble proteins that will be activated by enzyme-coupled receptors. Second messengers may or may not be involved with these receptors, though we are not going to explore any examples of second messenger activation by RTKs here (we already did that). We’re going to look at two new examples of common downstream effects: Ras and mitogen-activated protein (MAP) kinase signaling as well as another example of lipids that have roles in signaling.
Just like GPCRs, an activated receptor will need to relay the signal and amplify it somehow so that it can be spread around the cell and induce changes in the cell. We’ve already seen a couple of ways this can happen with GPCRs. In the case of RTKs, the first commonly activated molecule after receptor activation is a protein called Ras.
Ras is a molecular switch that is somewhat related to G-proteins. Molecular switches like these are very common in the cell. In fact, there is a very large family of these proteins (so big that we call it a superfamily) that is named after Ras. The members of the Ras superfamily of proteins are all small monomers (i.e., they are monomeric) and are all GTPases. This means that they convert GTP to GDP. We’ve seen many examples of GTPases in other chapters of this textbook, including the following:
The reason that we call these proteins molecular switches is that they have an “on” and “off” conformation, which is determined by the presence of GDP (off) or GTP (on). This is extremely similar to the G-proteins we saw earlier when discussing GPCRs. Like the G-proteins, Ras also has a small lipid tail covalently linked to it, which is exposed when the protein is activated. Thus, when the receptor activates Ras, it will act very similarly to a G-protein in that it will associate with the plasma membrane, diffuse laterally, and activate other proteins at the cell surface, as shown in Figure 07-13.
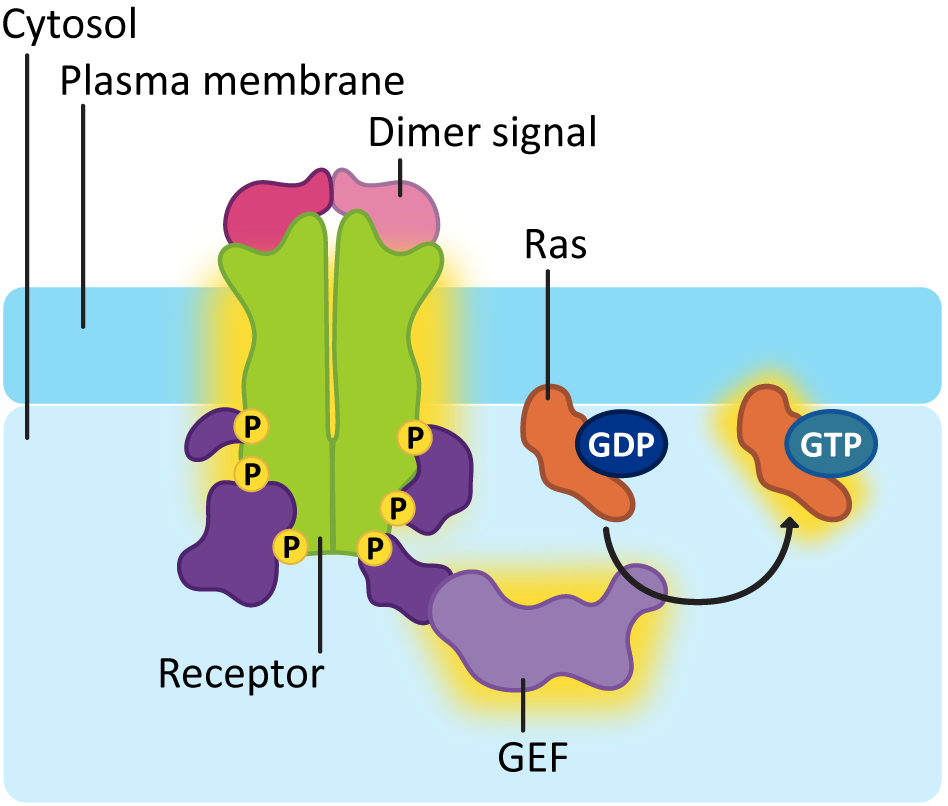
In order for Ras to be activated, a second enzyme known as a guanine-exchange factor, or GEF, is used to swap out the GDP for GTP. As you can see in Figure 07-13, the activation of the receptor allows the GEF to dock, which, in turn, makes sure it’s in the right spot to activate Ras.
Ras activation is also short lived, like G-proteins. The GTP that is bound to the activated Ras will eventually be hydrolyzed with the help of another protein known as GTPase-activating protein, or GAP. One of the more common downstream effects of Ras is that it activates a cascade of kinases that phosphorylate each other. You can think of it as very similar to a domino effect, where one domino knocks down the next, which knocks down the next, and so on. In this case, however, serine/threonine kinases are being activated in sequence, as each one phosphorylates the next. We call this a MAP kinase cascade (Figure 07-14).
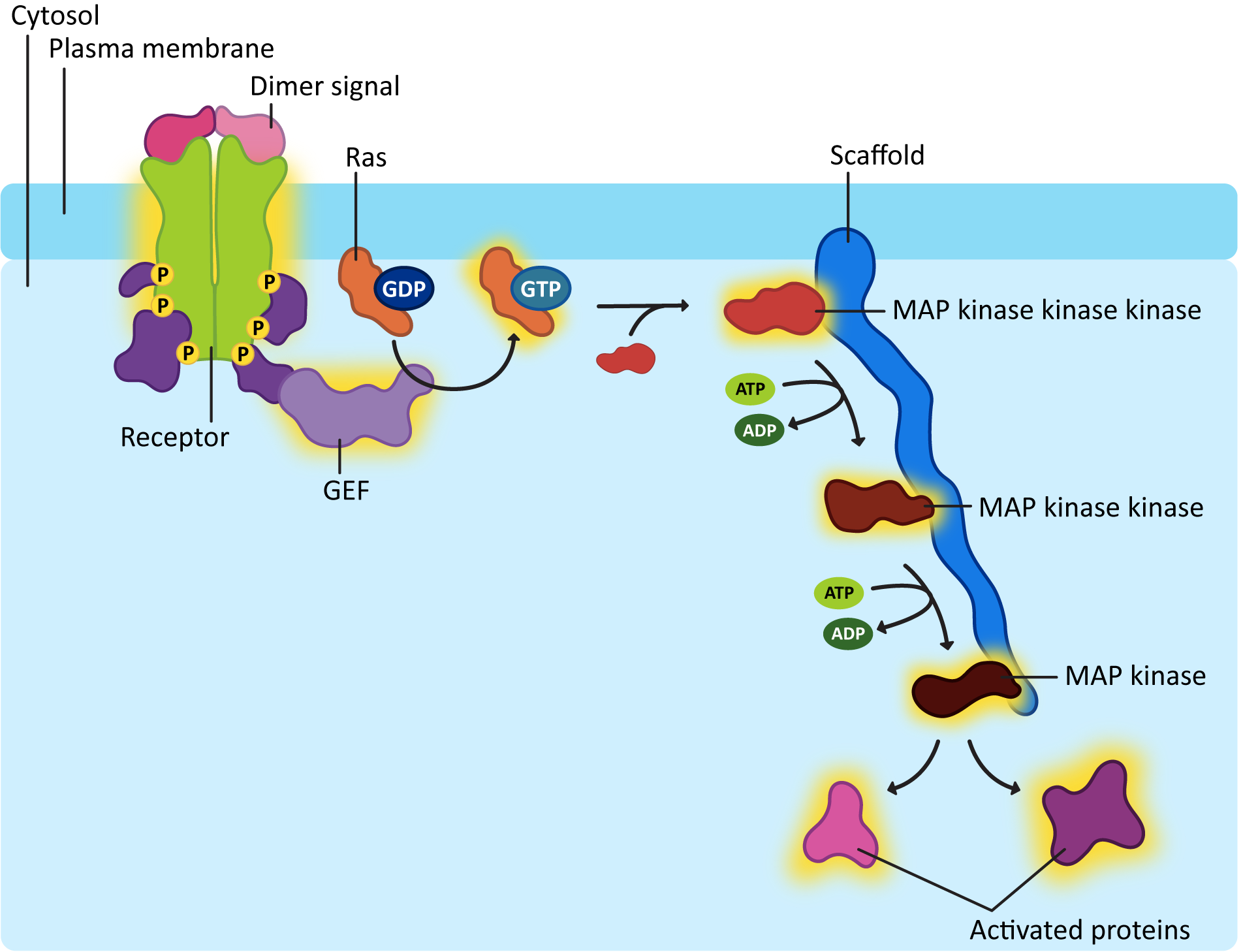
To be quite frank, MAP kinase cascades are very confusing. There are a lot of proteins involved, and the names are not really very creative, which makes things worse. Each of the kinases that gets activated in the cascade has the capacity to go on and activate many other proteins and enzymes, effectively amplifying the signal inside the cell. Usually, scaffold proteins help hold together the correct MAPK proteins. With so many of them in the cell and the fact that they are structurally similar, it would be easy to activate the wrong ones without taking specific measures to avoid that.
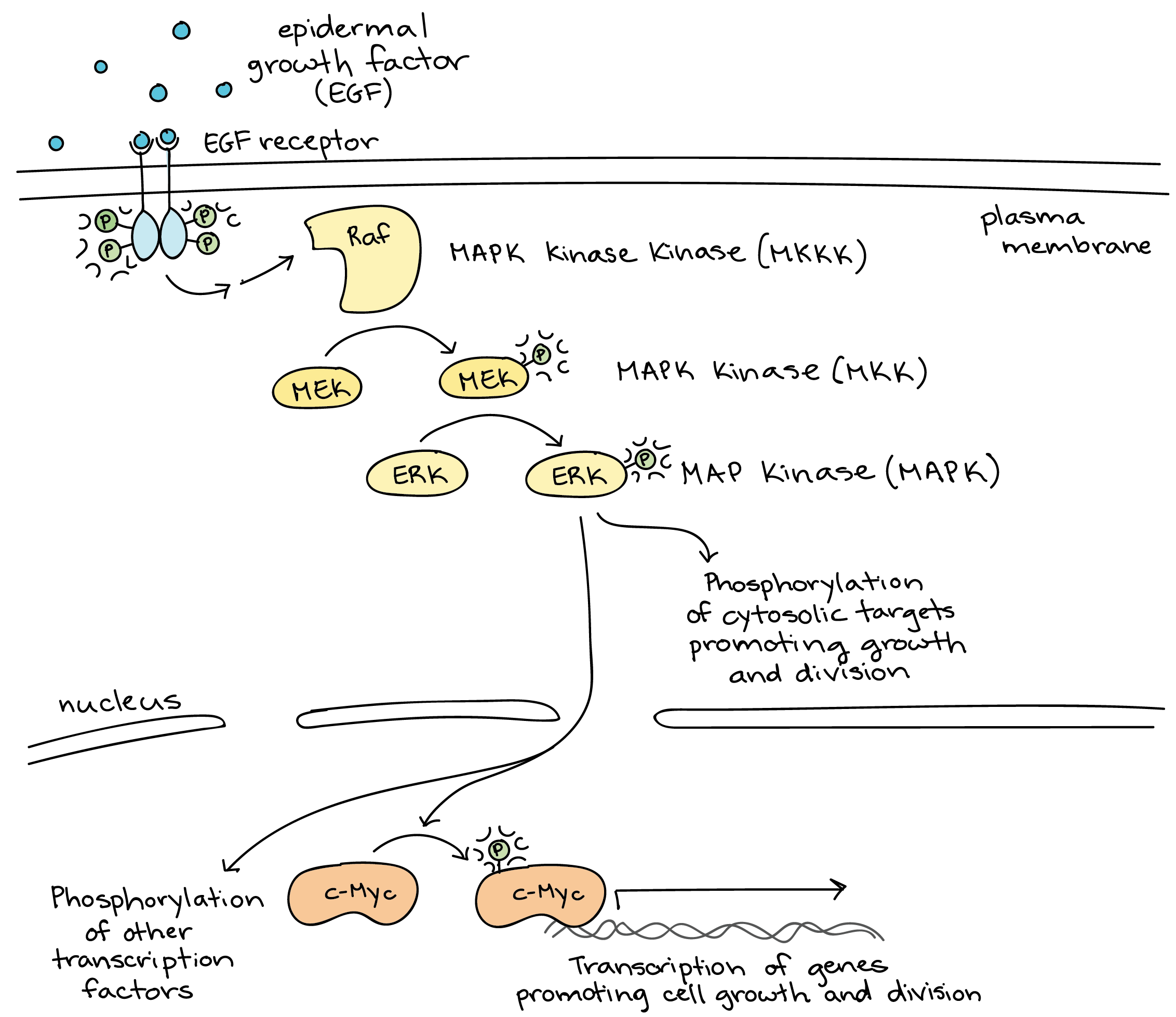
The downstream targets that get activated by a MAP kinase cascade will be involved in many different aspects of cellular function and are likely to have both fast and slow responses, much like all of the rest of the signaling we’ve seen so far. To show you how these cascades can be used, here is an example of a MAP kinase cascade simulated by the growth factor EGF (Figure 07-15). We are lucky to also have a video representation of this cascade to help us visualize what’s going on (Video 07-06).
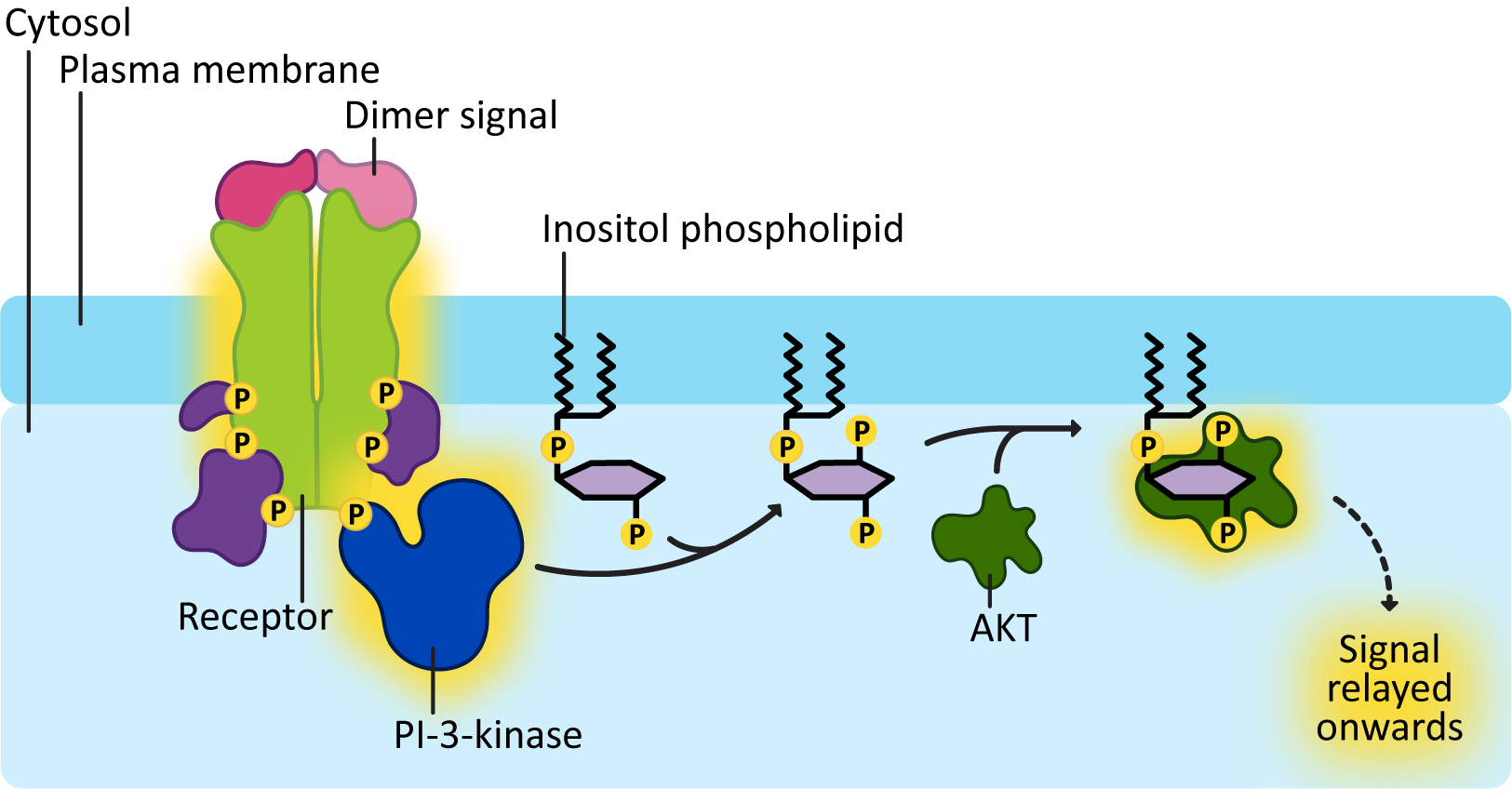
The addition of the phosphate at the third carbon results in the production of specific docking sites for a variety of proteins. For example, Figure 07-16 shows a protein called AKT docking on the modified PI molecule. AKT is a signaling protein specifically involved in cell survival and suppression of programmed cell death. PI-3-kinase gets involved in all kinds of cellular functions, from the formation of vesicle budding sites, to growth and proliferation, to cell motility.
The final point that is important to discuss, before we move on to experimental techniques, is a reiteration of how complex signaling really is. These pathways and mechanisms that we discuss do not function in isolation. Each and every cell on the planet is in a constant state of sending, receiving, and responding to signals from its environment. In fact, a cell that stops receiving signals from the environment for whatever reason will immediately die, as there will be nothing to stop programmed cell death (a.k.a. apoptosis) from occurring.
Very few signaling pathways are linear. They are more like an intricate and interconnected web, where each part can be influenced by many other parts. One signaling pathway could make another pathway easier or harder to turn on/off. To end this section, we offer an extremely simplified representation of a tiny subset of the signaling that can occur in a cell (Figure 07-17). We encourage you to explore and try to recognize the different parts of the signaling that we have explored in this chapter. Also look for the visual cues of activation and inhibition that we discussed at the end of Topic 7.1, which is indicated in the figure by either a pointed or blunt arrow.

Once you’ve done that, if you’d like to see a more realistic version of the complexity of signaling, the Cell Signaling Technologies website has an excellent set of signaling pathways that you can explore. (Hint: We recommend starting with insulin signaling or the regulation of actin dynamics, as they have most/all of the components we’ve explored, but there are lots of options.) It’s excellent practice to look at pathways you’ve never seen before and try to figure out what you can recognize. Once you start being able to recognize the various parts and proteins of a signaling pathway, it becomes a little bit less scary.
You may remember from earlier in the chapter that phosphorylation and dephosphorylation of proteins are very common posttranslational modifications that are used in signaling to regulate protein activity. You may also remember that phosphate groups are added to amino acids by kinases using ATP as the source of both the activation energy and the phosphate group. Most commonly, kinases will add phosphate groups on one of three different amino acid side chains: serines, threonines, and tyrosines (Figure 07-18). In signaling, phosphorylation of the amino acid residues in preexisting proteins allows for quick responses to external cues, which can be easily reversed by later removing the phosphate.
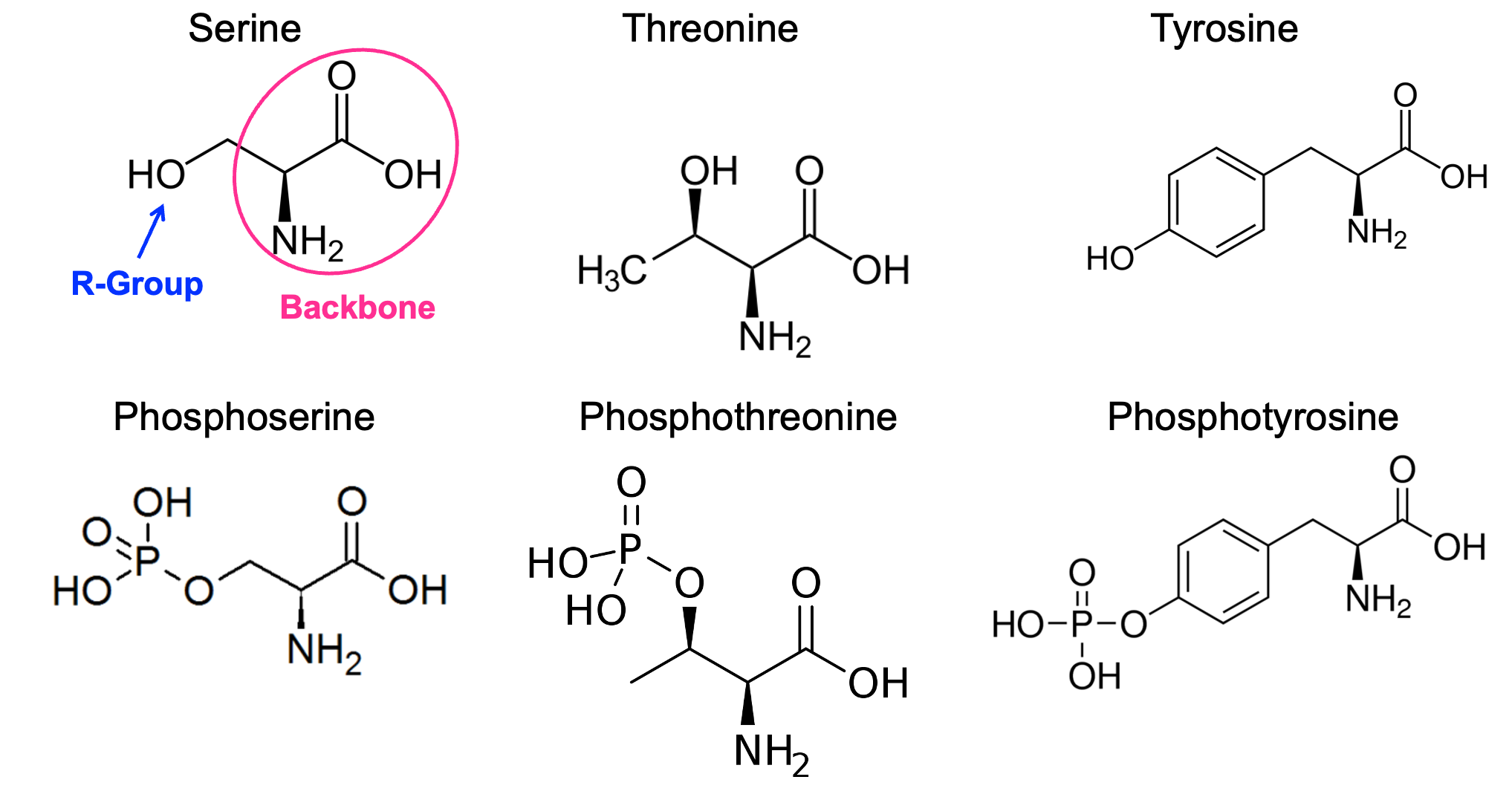
Signaling researchers spend quite a lot of time trying to understand the function of each phosphorylation site on a protein. Dissecting the contribution of an added phosphate to a protein’s overall activity is technically challenging. Bioinformatic analysis has given us the ability to know how many serines, threonines, and tyrosines are present in the protein sequence. However, it cannot tell us which of them are modified by kinases and what the effect of that modification would be on the function of the protein. That’s where experimentation comes in. We will explore two different approaches that are currently use by scientists: phosphomimetics and genetic code expansion (GCE).
In this approach, cell and molecular biologists genetically engineer proteins that have been modified in a particular way using a technique called site-directed mutagenesis. They can use site-directed mutagenesis to specifically replace an amino acid that would normally get phosphorylated with a different amino acid intended to “mimic” the phosphorylated state. Most commonly, negatively charged amino acids are used to replace the phosphorylated amino acid, such as aspartic acid (Asp) or glutamic acid (Glu). The negatively charged Asp and Glu residues are somewhat similar in size and charge to the phosphorylated amino acid, as can be seen in the example in Figure 07-19. The altered proteins have the added advantage that they cannot be dephosphorylated like a phosphorylated amino acid could be. As such, they remain in the “phosphorylated” conformation, which gives us a chance to study the effects of the “phosphorylated” protein in the cell.
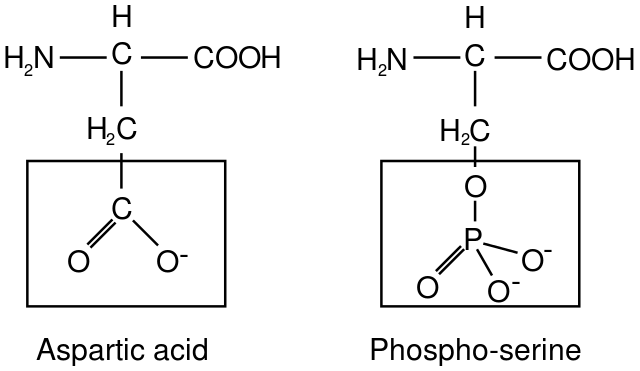
We can also mimic the dephosphorylated form of the protein by genetically engineering an alanine substitution at the site where a serine or threonine would have been originally. Since alanine cannot be phosphorylated, it blocks phosphorylation at that site and “mimics” the unphosphorylated version of the protein.
While these approaches have proven valuable in probing the biological function of phosphorylation of specific residues in some proteins, these approaches also have limitations. As you know from Chapter 2, the primary amino acid sequence determines folding and structure. Replacing one amino acid with another will never be a perfect replication of either the phosphorylated or unphosphorylated form of the original amino acid. The chemical properties of aspartic and glutamic acid are significantly different from serines, threonines, and tyrosines in a variety of ways, including electronegativity, volume, polarity, and hydrogen bonding. All of these chemical properties contribute to how a protein will fold and thus how it will function. Further, the nonphosphorylatable alanine amino acid has a much smaller R group than any of the amino acids it’s meant to replace, and since it’s nonpolar, it lacks their capacity to form hydrogen bonds. Thus, a different approach was devised.
To get around the problems that can arise with phosphomimetics, researchers created a new way to build phosphorylated versions of proteins without requiring the use of amino acids that “mimic” the original function. GCE is a technique that allows us to treat the phosphorylated version of the amino acid as its own entity within the genetic code so that we can insert it directly into the protein sequence. GCE is still a relatively new technique, but it is proving to be very powerful as we seek to learn more about posttranslational modifications, such as phosphorylation, and their role in protein function. Oregon State University is a world leader in this technique through their research center, the GCE4All Center.
The essence of this technique depends on how cells read and interpret the genetic code. The idea is that we “hijack” and modify how the cells interpret specific codons of the genetic code so that they now code for new, unique amino acids. Of course, the genetic code has 64 codons that code for 20 amino acids, and it is interpreted virtually identically across the entire tree of life. However, there are exceptions, most commonly in bacteria, where some codons are interpreted differently and code for different, unique amino acids. In these organisms, we have a different, expanded set of amino acids with which to create proteins. In GCE, we genetically engineer this exact same scenario to include specific new amino acids of our own choosing. For example, we could hijack a codon to code for phosphoserine instead of regular serine so that a phosphorylated version of the protein can be translated directly.
While the premise for GCE is relatively straightforward, hijacking the codons and making them code for a different amino acid requires careful manipulation of several components of the protein translation machinery. Remember that a key component of proper protein translation is the correct addition of the amino acid to the tRNA (for a refresher on translation, see this link from the Khan Academy). So if we want to change which amino acid a particular codon codes for, we must find a way to change which amino acid is attached to that codon. Adding the amino acid to a specific tRNA is done by a family of proteins known as amino acyl tRNA synthetases. Changing the genetic code begins with genetically modifying these synthetases so that the amino acid that is added to the tRNA is altered. Figure 07-20 provides a visual representation of this. In the figure, one of the stop codons (UAG) has been repurposed to code for phosphoserine (pSer in the figure). Once you have created this machinery, you can use site-directed mutagenesis to add your newly repurposed codon in any site in the genetic sequence where you want the new amino acid to be added, and the translation machinery will do the job for you. It’s worth noting that this system is mostly done in test tubes (i.e., in vitro), as changing the codon language used to create every protein in a cell would likely be detrimental and kill the cell.
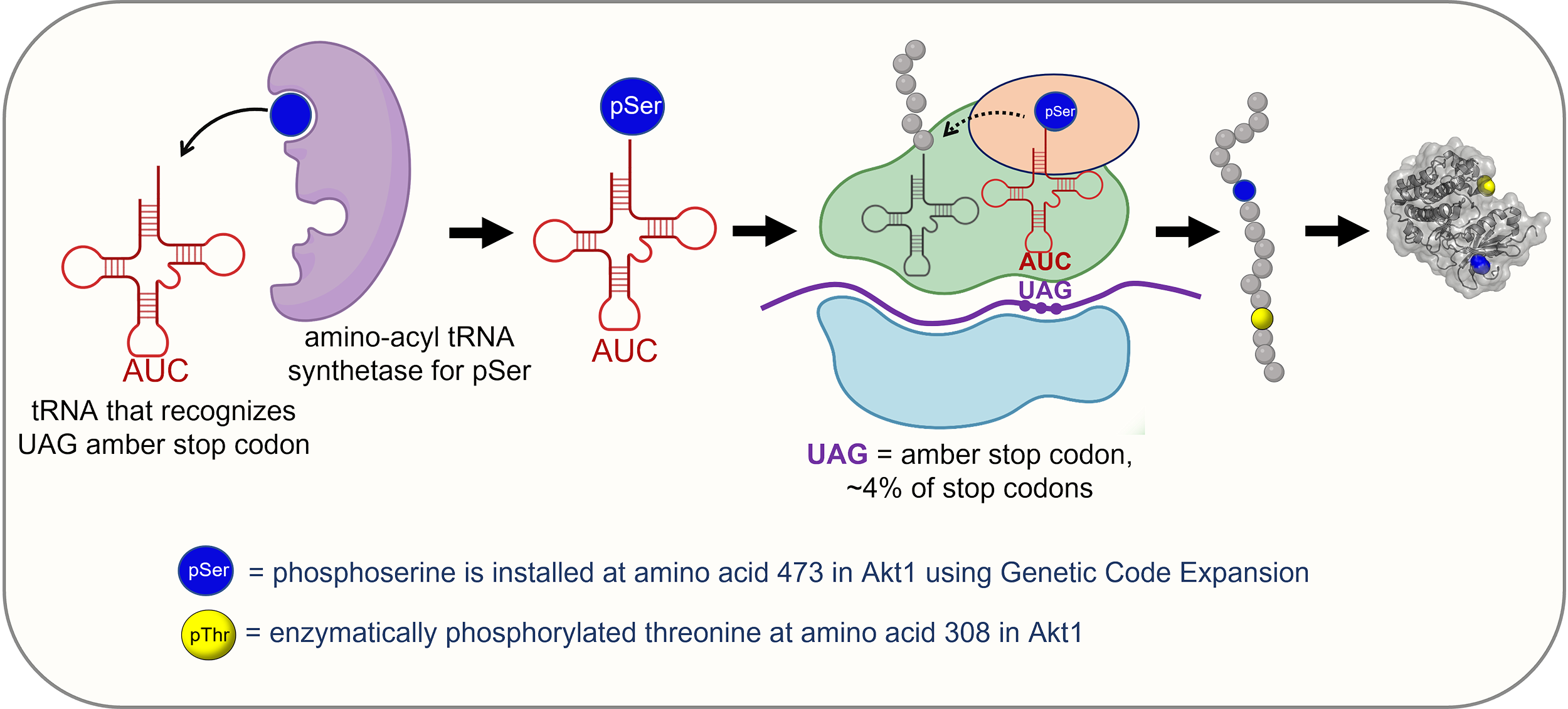
The advantage of this technique, compared to using phosphomimetics, is that you can add an actual phosphoserine rather than using a closely approximated amino acid, such as aspartic acid. Researchers do not know which kinases are involved in phosphorylation of a protein at a particular site. Using this method, they can create a phosphorylated version for use in vitro and then examine the protein’s activity by looking at its effect on known downstream targets.
GCE can be and has been used to address a wide variety of research questions that go far beyond the phosphorylation research described here. Many research areas have benefited from GCE, such as cell signaling, disease regulation, protein localization, protein-protein interactions, therapeutic development, and novel biomaterials and sensors. Its strength is in the fact that the novel amino acids that can be introduced into the genetic code are almost endless as long as the translation machinery can still work with them. They have been used to study topics such as posttranslation modification of proteins, linking two proteins together, adding on a stable fluorescent dye for microscopy in live cells, and other forms of labeling proteins to learn more about their structure. There are some examples of research that have been supported by Oregon State’s GCE4All Center, which gives you an idea of the versatility of this technique.
In this chapter, we explored how cells communicate and respond to their environment through cell signaling. In the first topic, we explained the fundamental anatomy of a signaling cascade, from the properties of a signal molecule, to the receptors to which it binds, to the intracellular signaling molecules, and finally to the types of responses that can be elicited from any given signal. Then we delved into specific examples of signaling systems and common pathways you might encounter in past and future coursework. Finally, we explored how we can use a couple of different genetic techniques to study how posttranslational modifications affect signaling cascades to understand the regulation of these complex systems. Hopefully, after reading this chapter, you will agree that this fundamental cellular process is as beautiful as it is complex.
Note on usage of these questions: Some of these questions are designed to help you tease out important information within the text. Others are there to help you go beyond the text and begin to practice important skills that are required to be a successful cell biologist. We recommend using them as part of your study routine. We have found them to be especially useful as talking points to work through in group study sessions.
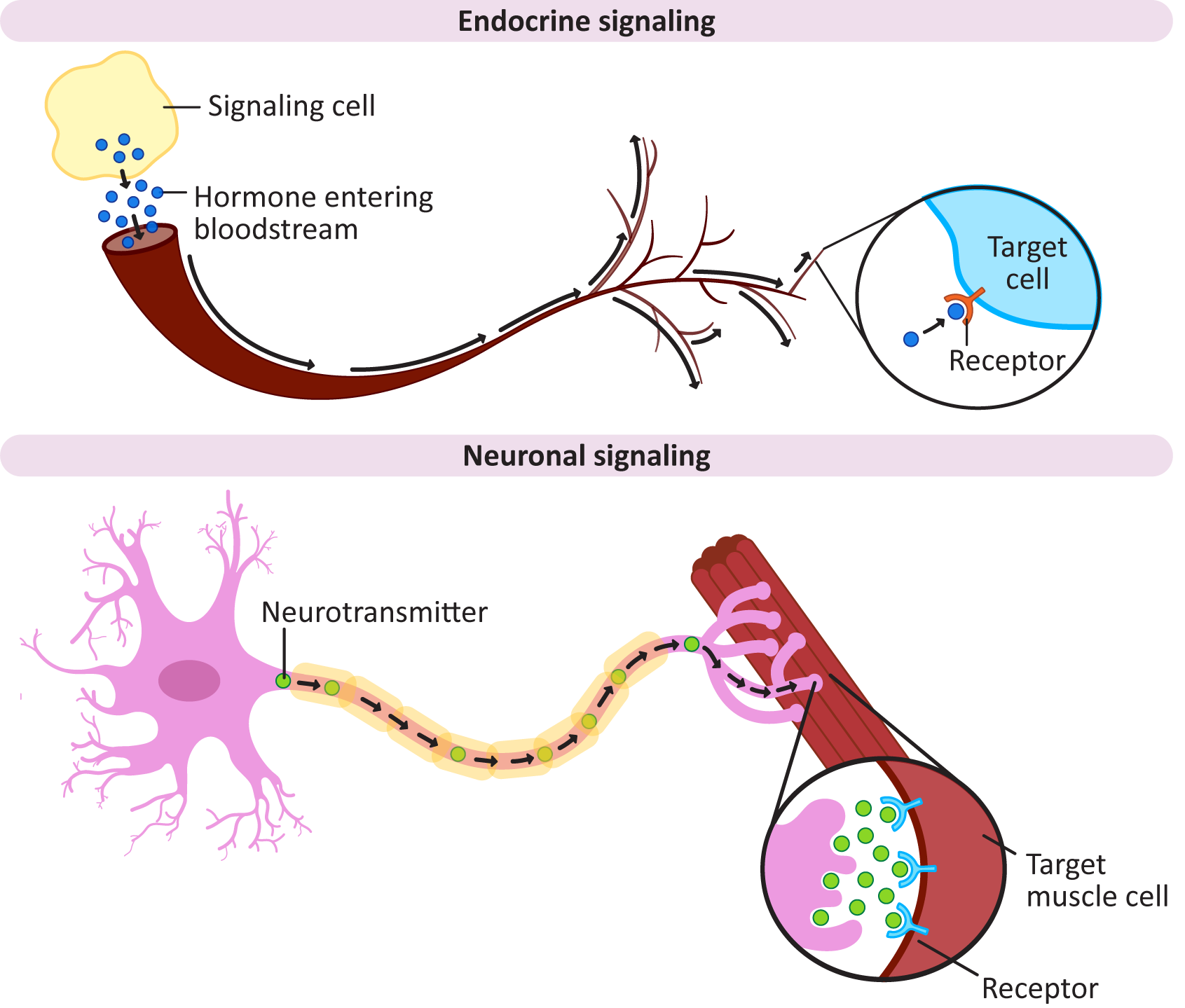
A long-range signaling pathway where a cell secretes a signaling molecule that is picked up by target cells not in the local vicinity.
× Close definitionReferring to neurons, an electrically excitable cell that is associated with the brain.
× Close definitionA short-range signaling pathway where a cell secretes a signaling molecule that is picked up by target cells in the local vicinity.
× Close definitionIn this type of signaling, the signal is released and received by the same cell.
× Close definitionIn this contact dependent signaling pathway, a cell attaches to another cell to initiate the signaling pathway.
× Close definitionA protein that binds a signal molecule to transmit a cascade intracellularly.
× Close definitionIn the context of proteins, refers to the favorability of a binding interaction. For instance, the protein when phosphorylated has greater affinity for its substrate. This means that the protein is likely to bind more tightly when the protein is phosphorylated.
× Close definitionA molecule that binds to a receptor to initiate a signaling cascade.
× Close definition“A class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior.” Source: Hormone. (2023, July 24). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Hormone
× Close definition“A signaling molecule secreted by a neuron to affect another cell across a synapse.” Source: Neurotransmitter. (2023, April 17). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Neurotransmitter
× Close definitionA regulated process of cell death.
× Close definitionThe chemicals that make up smell molecules. These are detected by odorant receptors in the nose. Subsequent signaling and activation of neurons to the brain help us sense these molecules.
× Close definition“In mammals, including humans…a signaling molecule in many physiological and pathological processes.” Source: Nitric oxide. (2023, July 11). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Nitric_oxide
× Close definition“A class of proteins responsible for sensing steroids, thyroid hormones, vitamins, and certain other molecules. These intracellular receptors work with other proteins to regulate the expression of specific genes thereby controlling the development, homeostasis, and metabolism of the organism.” Source: Nuclear receptor. (2023, May 25). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Nuclear_receptor
× Close definitionRefers to the process of signal transduction. When a signal is transduced, it is changed from its original form along the pathways. For example, when a signal binds to a receptor, this induces a conformational change in the receptor to activate it. The signal can be said to be transduced from a physical molecule to an activated receptor.
× Close definition“A series of chemical reactions that occur within a biological cell when initiated by a stimulus.” Source: Biochemical cascade. (2023, June 16). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Biochemical_cascade
× Close definition“The process by which a chemical or physical signal is transmitted through a cell as a series of molecular events.” Source: Signal transduction. (2023, June 20). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Signal_transduction
× Close definitionThe attachment of a phosphate group to a biological molecule. When applied to a protein, this can often change the conformation of the protein, resulting in a functional change.
× Close definitionThe process of removing a phosphate chemical group from a protein.
× Close definitionProteins that “interact and/or bind with multiple members of a signaling pathway, tethering them into complexes. In such pathways, they regulate signal transduction and help localize pathway components (organized in complexes) to specific areas of the cell.” Source: Scaffold protein. (2021, September 17). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Scaffold_protein
× Close definitionBiological molecules released as part of a signaling cascade as a way to amplify the signal.
× Close definitionA regulatory mechanism whereby the product of a process will influence (upregulate or downregulate) itself.
× Close definitionA regulatory process where a receptor is brought into the cell via endocytosis, and then degraded in the lysosome.
× Close definitionA regulatory process where a receptor is endocytosed such that it isn’t available to bind to signal molecules on the cell’s surface.
× Close definitionA regulatory process where a biological molecule directly inhibits the cell receptor’s function.
× Close definitionWhen a biological molecule that is part of a signaling cascade is prevented from participating in its role.
× Close definitionThe region 5′ of the indicated location in a piece of DNA.
× Close definitionRefers to the region of the DNA that is 3′ of the area referenced. For example, in a sentence, you could say, “The transcription stop site is located downstream of the transcription start site.”
× Close definitionWhen a signal binds to this type of receptor, an ion channel is opened as a result.
× Close definition“An inositol phosphate signaling molecule. It is made by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, by phospholipase C (PLC). Together with diacylglycerol (DAG), IP3 is a second messenger molecule used in signal transduction in biological cells.” Source: Inositol trisphosphate. (2023, May 1). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Inositol_trisphosphate
× Close definition“Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins.” Source: Phosphatidylinositol 4,5-bisphosphate. (2023, January 26). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Phosphatidylinositol_4,5-bisphosphate
× Close definition“A large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G-proteins.” Source: G-protein-coupled receptor. (2023, April 16). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/G_protein-coupled_receptor
× Close definition“A family of proteins that act as molecular switches inside cells and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP).” Source: G-protein. (2023, July 21). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/G_protein
× Close definition“A second messenger, or cellular signal occurring within cells, that is important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway.” Source: Cyclic adenosine monophosphate. (2023, April 30). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate
× Close definition“A glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages.” Source: Diglyceride. (2022, November 2). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Diglyceride
× Close definitionAn enzyme responsible for making cAMP (cyclic AMP) from ATP. Also called adenylate cyclase.
× Close definitionAn enzyme that cleaves a phosphodiester bond. These occur in between nucleic acids or within a ringed nucleotide such as cyclic AMP.
× Close definition“A family of serine-threonine kinase whose activity is dependent on cellular levels of cyclic AMP (cAMP).” Source: Protein kinase A. (2023, July 23). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Protein_kinase_A
× Close definition“Phospholipase C’s role in signal transduction is its cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers.” Source: Phospholipase C. (2023, June 24). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Phospholipase_C
× Close definitionA regulatory mechanism whereby the product of a process will negatively influence (downregulate) itself.
× Close definitionKinases (enzymes that add a phosphate group) on serine or threonine amino acids of the target protein.
× Close definitionAn enzyme that adds a phosphate chemical group to another enzyme. These can be added on serines, threonines, or tyrosine.
× Close definitionProteins that remove a phosphate from a target protein.
× Close definition“The high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones.” Source: Receptor tyrosine kinase. (2022, February 19). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Receptor_tyrosine_kinase
× Close definitionThe process of two biomolecules binding.
× Close definitionProtein locations where other biomolecules are able to bind.
× Close definition“A family of related proteins that are expressed in all animal cell lineages and organs. All Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells.” Source: RasGTPase. (2023, March 17). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Ras_GTPase
× Close definition“Proteins can switch between active and inactive states, thus acting as molecular switches in response to another signal. For example, phosphorylation of proteins can be used to activate or inactivate proteins.” Source: Molecular switch. (2023, June 10). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Molecular_switch
× Close definitionExisting as a single unit. With a protein, this refers to having a single polypeptide chain.
× Close definitionA family of proteins that will swap a GDP for GTP on a G protein.
× Close definitionA family of proteins that helps a G protein accelerate its GTPase activity (converting GTP into GDP).
× Close definitionA three-part signaling cascade where a MAPKKK phosphorylates a MAPKK, which then phosphorylates a MAPK. These are part of a larger signaling pathway.
× Close definition“A family of related intracellular signal transducer enzymes capable of phosphorylating the 3 position hydroxyl group of the inositol ring of phosphatidylinositol (PtdIns).” Source: Phosphoinositide 3-kinase. (2022, October 27). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Phosphoinositide_3-kinase
× Close definitionA ringed sugar molecule that is commonly used in signaling pathways.
× Close definitionAn amino acid substitution in a engineered protein to mimic the rough charge and shape of a phosphorylated protein at that location.
× Close definitionA biochemical technique to encode designer amino acids into genetically engineered proteins.
× Close definitionResponsible for putting the amino acid on the tRNA.
× Close definitionFundamentals of Cell Biology Copyright © 2024 by Lauren Dalton and Robin Young is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, except where otherwise noted.